Glimpse of the Clinical Study Outcome
Efficacy and Safety of aspurūs ® Shatavari Root Extract for the Management of Menopausal Symptoms: A Double-Blind, Multicenter, Randomized Controlled Trial
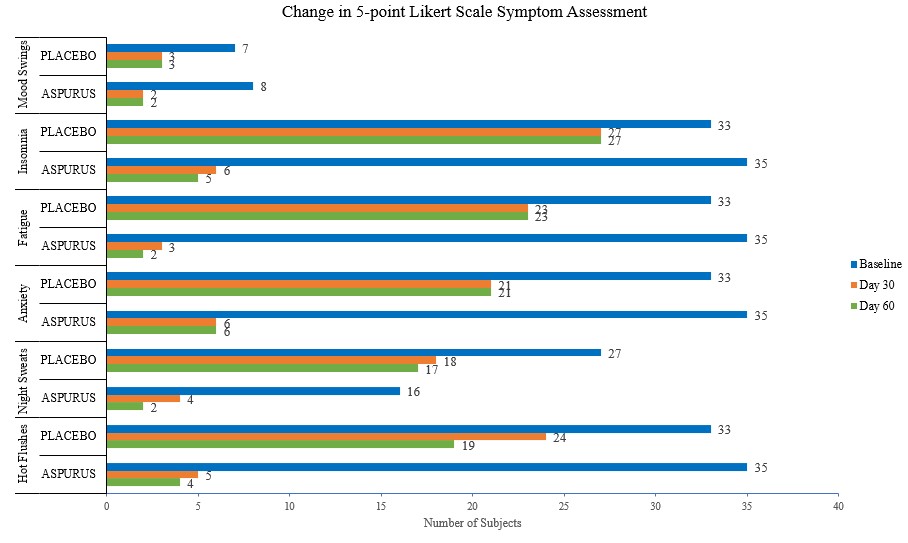
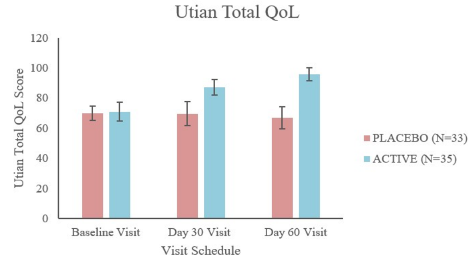
A randomized, double-blinded, multi-center, placebo-controlled gold standard clinical study was conducted to investigate the use of our patent pending full-spectrum Shatavari root extract on 70 menopausal (peri, pre-and post) women between the ages of 40 to 65 years undergoing the emotional and psychological changes associated with menopause along with menopausal symptoms such as hot flashes, night sweats, anxiety, fatigue, depression, insomnia, and mood swings. The study was conducted for 8 weeks aimed to investigate the safety and efficacy of aspurūs® with the placebo in the management of menopausal symptoms and regulation of the HPO axis.